Alzheimer’s Dementia is medically characterized as a short list of things: progressive cognitive decline, hyperphosphorylation of tau proteins, and the buildup of hard-to-rid-of beta-amyloid peptide fragments, especially Aβ42 (an extremely easily-clumped species). Creation of Aβ42 is pathologically caused by the cleavage of amyloid precursor proteins (APP) by β-secretase enzymes outside of the Aβ region of the protein. Normally, when α-secretase cleaves this protein, it “destroys” this “stickier” region. However, β-secretase cleaves amyloid precursor proteins outside of this “stickier” region, essentially keeping it intact. After cleavage, γ-secretase is the enzyme that is responsible for “releasing” the cleaved fragments into the extracellular space. For a generalized understanding, the production of these isn’t the main problem in AD. It’s that the enzymes, which are responsible for “ridding” of them, are expressed in a lesser frequency, or are broken down due to oxidative stress. In other words, there is so much radioactive waste that your local garbage facility is starting to malfunction.
Here is a picture for better understanding:
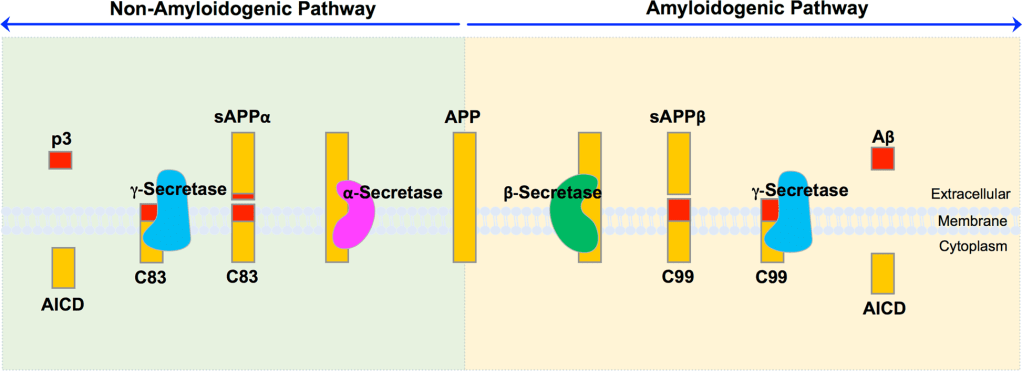
Hur, J.-Y. (2022). γ-Secretase in Alzheimer’s disease. Experimental & Molecular Medicine, 54(4), 433–446. https://doi.org/10.1038/s12276-022-00754-8
*Note (intricacies): The main difference between these pathways is in the location of the initial cleavage. The Aβ region spans both the extracellular domain and the transmembrane domain of APP. There is a portion “poking out” of the cell. α-secretase cleaves within this region, irreversibly disrupting the hydrophobic (sticky) Aβ sequence. In contrast, β-secretase cleaves immediately N-terminal to the Aβ region, leaving the full sequence intact. γ-secretase then performs intramembrane cleavage near the extracellular boundary, defining the length of Aβ (e.g., Aβ40 vs. Aβ42) and releasing the peptide into the extracellular space.
Phosphorylation is normally used for protein regulation; one could even call it a generalized protein mechanic and on/off switch, 2 in 1. Protein kinases add phosphate groups onto specific amino acids that make up the protein. Tau is an essential protein related to supporting the microtubule structures within the cell. Think of microtubules as the “highway” of a cell, essential for the transportation of vesicles and molecules. In the case of AD, the excessive addition of these phosphate groups leads to a “deformed” structure and detachment from the microtubules, forcing them to malfunction and form neurofibrillary tangles, where, just like the beta-amyloid plaques, a build-up of these malfunctioning pieces starts to prove detrimental to neural cells.


Leave a comment